Beating dengue at its game
By Dr Audrey Khoo, Science writer and Dr Chua Li Min, Science writer
The dengue virus is on the move. Like a player in a game of Risk, its aim is simple—to attack and conquer territories on the world map—not with the roll of the dice, but by piggy-backing the Aedes mosquito.
That’s why, unlike the recent threat posed by the SARS-CoV-2 coronavirus, this virus cannot be thwarted with safe distancing restrictions and mask wearing. And with global temperatures heating up due to climate change, the scales are tipping in the virus’ favour.
“Previously the mosquitoes were confined to the tropics. Now they’re beginning to encroach into the subtropics,” said
Professor Ooi Eng Eong from Duke-NUS’
Emerging Infectious Diseases Programme who has been studying the disease for more than 20 years.
“The projection is that by 2070, more than two thirds of the world’s population will be living in places at risk of dengue,” he added, counting the increase in travel for trade and leisure as another reason for the spread of the mosquitoes and, consequently, the virus.
.jpg?sfvrsn=4c8a18f4_0)
Professor Ooi Eng Eong has been studying dengue for more than 20 years
For patients afflicted by dengue fever, the only course of treatment is to manage the symptoms. While most make a full recovery, a small group develop a deadlier form of the disease, also known as dengue haemorrhagic fever.
“[That’s when] your blood vessels become leaky and fluid starts leaking,” said Ooi. “Then your blood volume shrinks and your heart has to work a lot harder to keep your blood pressure within the normal range.”
To compensate for this loss, patients are usually given fluids intravenously. This process is a delicate balancing act and one that requires the watchful eye of an experienced clinician. Because the outlook can be grim if the patient’s condition is not carefully managed.
“Once this period of leakage ends, recovery is very fast. When that happens, the body starts pulling all that extra fluid back into the bloodstream,” added Ooi. “If you don’t stop putting in fluids at this point, the patient can go into fluid overload as some of the fluid starts going into the lungs. All of a sudden, they cannot breathe and patients can literally die from this.”
And it is not only the expanding habitat of the Aedes mosquito that is concerning. Over the last 15 years, Ooi has seen an uptick in the number of elderly patients with dengue—a worrying trend as older patients are more susceptible to severe dengue. Besides their advanced age, these individuals also tend to suffer from health complications caused by chronic diseases such as diabetes and heart diseases.
“Ideally, it would be best to have a drug that can knockout the dengue virus early so we can reduce the viral burden in patients to prevent these complications. Unfortunately, right now there’s no drug that’s licensed for such use.”
With limited options for vaccines and no therapeutics available, the projected increase in the dengue burden is a global health concern that Duke-NUS researchers like Ooi hope to tackle.
Not all dengue viruses are the same
What makes the search for treatments and vaccines all the more challenging is that while all dengue viruses wreak havoc on the body, they are not neutralised by the same antibodies. This has resulted in four distinct serotypes of the virus: DENV-1, DENV-2, DENV-3, and DENV-4.
“It is helpful to think of the four serotypes of dengue viruses as four different viruses. Genetically, the distance between DENV-1, DENV-2, DENV-3, and DENV-4, is comparable to the genetic distance between, for example, the Japanese encephalitis virus and the West Nile virus,” explained Ooi.
This means that an individual can potentially suffer four episodes of dengue infections without the benefit of any pre-existing immunity.
And that is not all.
“The clinical observation is that the second infection is often worse than the first. This happens when the antibodies developed from the first infection are unable to neutralise the virus from the second infection,” he added.
To make matters worse, existing antibodies from the first infection can go rogue, helping the invading virus infiltrate immune cells undetected.
Zooming in on the virus
Part of the reason why an effective vaccine and treatments have been so elusive lies in the shape of the virus, believes
Professor Lok Shee Mei, a colleague of Ooi’s with the Emerging Infectious Diseases Programme.
“Even a single amino acid mutation can result in a very different virus structure,” said Lok, causing the virus to appear “smooth, bumpy and sometimes even club-shaped”.
.jpg?sfvrsn=8f3e5ceb_0)
Professor Lok Shee Mei studies the structure of viruses including dengue and Zika
By studying the virus’ structure and how it changes, Lok and her team hope to decode its inner workings. Inner workings that can have significant consequences for the world outside the mosquito.
“[Studying the structure] enables us to detect escape mutants that may be circulating in the environment, knowledge of which may come in handy when designing vaccines or boosters,” said Lok, who is also the NUS Provost's Chair Professor.
Lok’s fascination with the structure of the dengue virus began with her studies of a protein on the surface of the virus known as the viral envelope or E protein, which is an important component on the virus that enables it to interact with host cells. Using a technique known as X-ray crystallography, Lok zoomed in on the E proteins by growing crystals of them to visualise these proteins as well as their interactions with neutralising antibodies known to bind to them.
“It’s like a diamond, except it’s much more expensive because it is so difficult to make,” said Lok, referring to the process of crystal growing. After shooting X-ray beams onto the crystals, she would then compute the three-dimensional structure that forms when the dengue E protein and human antibodies targeting it are mixed together.
While Lok was comparing the X-ray crystal structure of this combination with the available cryogenic electron microscopy (cryoEM) structure of the naked whole dengue virus, she discovered that her E protein structure showed an important difference. From her experiments she knew that the human antibody could bind to the E protein and prevent infection. But in the whole dengue virus, which had been grown at temperatures ranging from 28oC to 30oC to mimic the body temperature of a mosquito, the region of the protein where the antibody docks stayed concealed.
That was when she realised that the dengue virus exhibited different structures at varying temperatures. At a human’s physiological temperature of 37oC, the virus surface might be more active, enabling the regions recognised by the antibody to be transiently exposed. To investigate her hypothesis further, Lok’s team examined the structure of the dengue virus that had been incubated at 37oC using cryoEM. True enough, the viruses morphed from smooth particles to bumpy particles as they moved from an environment akin to that in the cooler vector (the Aedes mosquito) to that of a warm-blooded human host.
“Knowing how the virus structure changes as it enters a human host may be useful when it comes to designing therapeutics against the virus, as it can help predict which antibodies may bind efficiently to the virus or which sites can be targeted to inhibit the virus replication process.”
Professor Lok Shee Mei
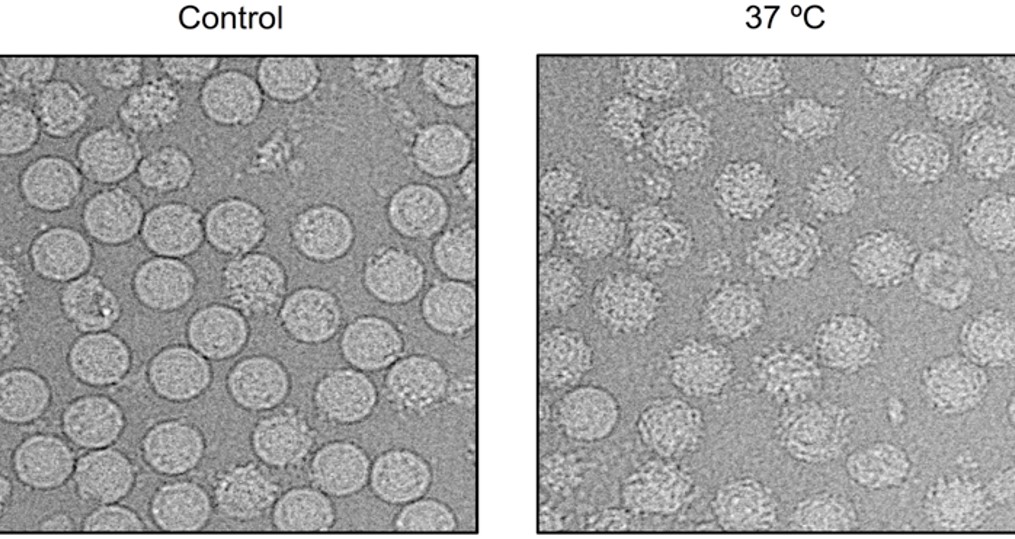
Structural changes seen on the dengue virus as the viruses move from an environment akin to that in the cooler vector (the Aedes mosquito) (left) to that of a warm-blooded human host (right) // Credit: Lok Shee Mei & Guntur Fibriansah
Designing a plan of attack
At the same time, Ooi’s team embarked on a mission to understand what makes a good vaccine by examining the well-established yellow fever vaccine, which uses a weakened form of the virus to generate an immune response to the pathogen.
“It has helped us to understand why some people get sick from viral infections. We were able to identify which processes—host responses and innate immune responses have to be switched on to provide good immunity from vaccination,” said Ooi.
“So we identified a set of genes that have to be turned on and off at various time points after you receive a vaccine. Now we can tap that knowledge and use it to understand how other vaccines are working and how they can be improved,” he added.
Drawing on this experience, Ooi is collaborating with biopharmaceutical company, Takeda to understand the inner workings of their dengue vaccine candidate, which makes use of an attenuated DENV-2 virus strain as the backbone of the vaccine.
Through “cutting and pasting” or splicing structural genes for the remaining serotypes—DENV-1, 3 and 4—into this backbone, the vaccine aims to protect against the four dengue serotypes as it stimulates the immune system to produce different types of antibodies. The DENV-2 backbone also leads to the activation of another group of immune cells known as T cells, enhancing the repertoire of immune cells that are primed to respond to the virus in the face of an actual attack.
Named TAK-003, this dengue vaccine candidate has recently completed phase III clinical trials and long-term follow-up studies, with promising results. It reduced hospital admissions for dengue by 84 per cent and symptomatic infections by 61 per cent. And importantly, no important safety risks were identified.
“The long-term tetravalent immunisation against dengue efficacy study results indicate that TAK-003 could be an important addition to the limited tools we have to prevent dengue, particularly given the demonstrated protection against hospitalisations,” Ooi had said in press release issued by Takeda on 9 June.
With the regulatory review for TAK-003 underway in the European Union and select dengue-endemic countries, Ooi is excited about what is to come.
“I hope it’ll be licensed. Without a vaccine, we’re just going to be facing more and more dengue cases. But having a vaccine will certainly prevent the projected increase in the dengue burden,” he said.
Fighting for the future
Meanwhile at the newly launched Satellite Centre at Duke-NUS, work on developing a therapeutic for dengue and its flavivirus cousins such as Zika has also begun.
The Satellite Centre at Duke-NUS, which is part of Johnson & Johnson (J&J) Centres for Global Health Discovery network and its only outpost in Asia so far, marks the latest effort of the SingHealth Duke-NUS Academic Medical Centre to develop a collaborative Discovery District on its campus that encourages seamless collaboration between academia, healthcare and industry to boost bench-to-bedside research.
Dr Milly Choy, a senior research fellow at Duke-NUS who manages the new centre as well as the School’s mosquito-breeding facility (which she talks about in this podcast), said: “We will zero in on antiviral profiling of novel compounds against flaviviruses in this region, and establish a new drug discovery programme by exploring conserved antiviral drug targets and developing a strategic plan for host targets.”

Dr Milly Choy (right) speaks about the work of the new centre during its official launch held on 21 June at the Academia
“Using state-of-the-art approaches in molecular virology, and innovative in vivo approaches to evaluate candidate drugs in model systems, we hope to develop a drug pipeline for dengue, as well as other flaviviral diseases,” added Dr Kitti Chan, a senior research fellow at Duke-NUS and a lead scientist with the Centre.
Besides the new drug discovery programme, the team at Duke-NUS is also collaborating with SingHealth’s Investigational Medicine Unit to conduct a phase IIa clinical trial evaluating Janssen’s antiviral compound for the prevention and treatment of dengue.
With vaccines and drugs in the pipeline to level the playing field, Ooi is hopeful.
“I don’t think one single tool is going to solve this problem, so vaccines or therapeutics alone are not going to be the magic bullet, but when used in conjunction with other disease preventive tools such as Project Wolbachia to try and suppress transmission, that gives us a fighting chance.”

Studying the Aedes mosquito up-close in the Duke-NUS insectary