Going back to basics reveals new uses for old drugs
By Dr Chua Li Min, Science writer
Dr Madhulika Tripathi (left) with Professor Paul Yen (centre) and Assistant Professor Brijesh Singh
Most patients in Assistant Professor Tan Hong Chang's clinic don’t just have high blood sugar levels. They also suffer from complications including high fat levels in their blood. For these patients, Tan may add, to a prescription of a healthier lifestyle an oral medication called fenofibrate.
Known to be effective at enhancing the breakdown of fats in the body, the prescription of fenofibrate for patients with high cholesterol is a standard clinical decision that has been practised for decades. But this almost 50-year-old drug remains a bit of an odd ball: it first emerged from the race to tackle what had, by the 1950s, become a major health problem yet its mechanism of action is still not fully understood.
And fenofibrate is not alone. There are many drugs—and even nutritional supplements—whose mechanisms are not fully understood, providing researchers with opportunities to bring new treatments to patients faster and without the hefty price tags of new medicines.
A collaboration in the making
Keen to understand exactly how fenofibrate benefits diabetic patients, Tan, who is the research director at the SingHealth Duke-NUS Diabetes Centre, started studying the drug’s effectiveness against diabetes-related complications, including diabetic kidney disease and diabetic retinal disease.
Bouncing ideas off his colleague, Tan talked through his latest research with Associate Professor Liu Yu-Chi, a fellow clinician-scientist from the Singapore National Eye Centre.
“So I was telling her about how the drug has also been used to treat diabetic eye disease, where abnormal blood vessels grow in the retina”, said Tan.
“And that got me really excited,” added Liu, an associate professor with the SingHealth Duke-NUS Ophthalmology and Visual Sciences Academic Clinical Programme.
Combing the existing literature on the drug and its mechanism, Liu soon came across a study about fenofibrate preventing the degeneration of nerves in the body.
That made her wonder if the drug could exert the same beneficial effect on nerves at the front of the eye, where the cornea sits. “In diabetic patients who experience defects in metabolism, degeneration of the corneal nerves results, causing diabetic corneal neuropathy,” she explained.
She spoke to Tan about her idea. “There was some basis in her reasoning, so I said, ‘Okay, let’s take a look at it’,” recalled Tan on how their joint study first began. “So the collaboration was a natural fit and fairly straightforward.”

Associate Professor Liu Yu-Chi (left) with Assistant Professor Tan Hong Chang // Credit: Liu Yu-Chi
The quest to repurpose an existing drug
The duo invited a small group of patients whom Tan was following up with from DYNAMO (the Diabetes Study in Nephropathy And other Microvascular Complications)—a study targeted at reducing the prevalence of diabetic kidney disease—to have their corneas scanned after treatment with fenofibrate.
“We picked up that there’s actually some signs of nerve regeneration following treatment,” said Tan, who is also with the SingHealth Duke-NUS Medicine Academic Clinical Programme.
To corneal specialist Liu, this was exciting. “Up to 64 per cent of patients suffer from diabetic corneal neuropathy during the clinical course of their disease,” she explained.
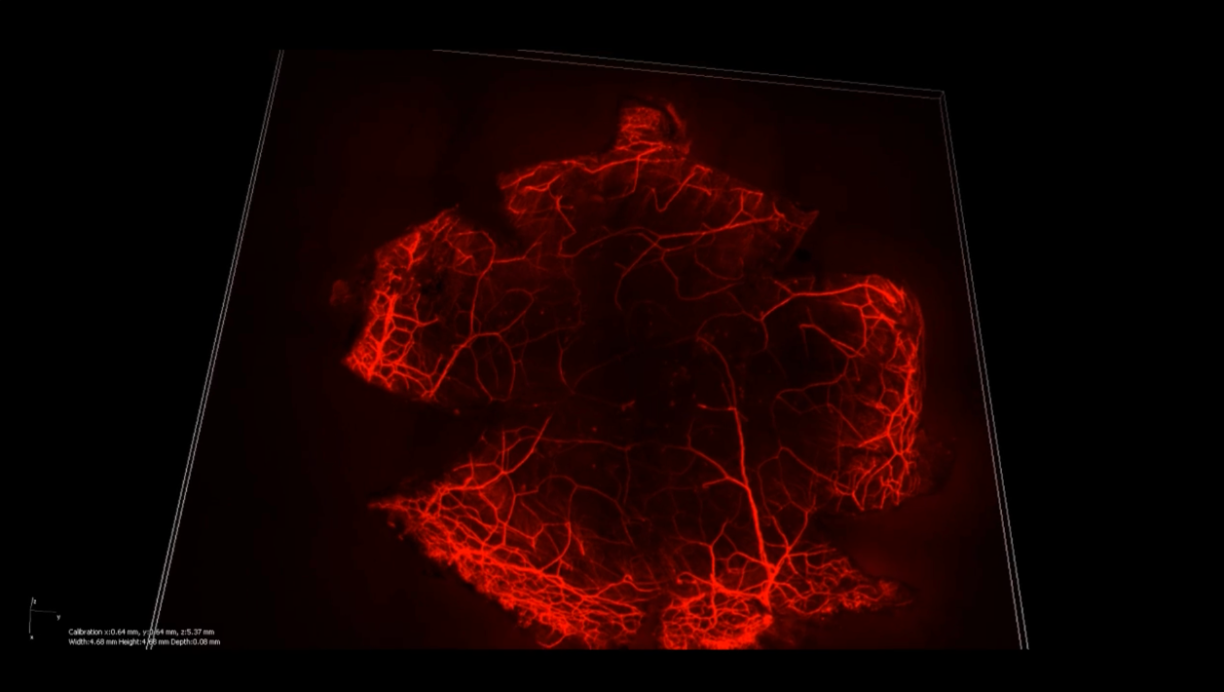
An image showing stained corneal nerves // Credit: Liu Yu-Chi
“The symptoms usually start off mild, characterised by dryness in the eyes, or a gritty sensation that can worsen if left untreated,” said Tan.
In the most severe cases, patients who have lost all sensation in the eye suffer injuries without realising it. “They have to be treated for eye infections or even perforations to the cornea,” added Liu.
But their findings could soon bring change for these patients.
“Now we have a candidate drug. So we can focus on finding out the best way to make this work,” said Tan.
“And the best thing is, we know that this drug is safe compared to investigative drugs without a known safety profile.”
Assoc Prof Liu Yu-Chi
“And the best thing is, we know that this drug is safe compared to investigative drugs without a known safety profile,” said Liu, thereby cutting the development time from the typical 10 to 15 years.
“Compared to a completely new drug, older drugs are also more affordable due to the availability of generic versions. And patients also benefit from the faster regulatory approval process.”
Asst Prof Tan Hong Chang
Have a question? Send it in and it may be answered in the next issue of MEDICUS!
ASK MEDICUS
Uncovering the hidden talents of natural compounds
While Tan and Liu found their route to innovation by starting with a drug with unknown mechanism, Professor Paul Yen and his team from Duke-NUS’ Cardiovascular and Metabolic Disorders Programme approached the problem from the other side of the coin—scrutising the mechanism of action in their disease of interest.
As part of their research, Yen’s group has been trying to find ways to help patients with non-alcoholic fatty liver disease (NAFLD).
“About 40 per cent of Asians and Singaporeans have NAFLD, and it can progress to a severe form of disease, known as non-alcoholic steatohepatitis or NASH, where the liver gets severely inflamed and damaged due to an accumulation of fats,” said Yen. “A small percentage of these patients will develop liver failure that ultimately requires transplantation.”
With no known pharmacological cure, the only means of treatment involves reducing the liver’s fat content through lifestyle changes. “But most patients are not very disciplined when it comes to exercise and dieting. So having a drug therapy will help improve their liver condition,” said Yen.
But first, his team had to figure out what was going on behind the scenes to cause the accumulation of fat in the liver. “As we understand the mechanisms better, we may be able to identify existing compounds that could be useful in NASH,” stressed Yen.
When he came across a study highlighting the importance of the body’s internal trash disposal system—a process called autophagy—in breaking down fats, Yen knew they had to start looking in that direction. If the body’s process of breaking down fats through autophagy was defective in the liver, that would explain the high levels of fat seen in NASH patients.
The final piece of the puzzle
Following that trail, the team’s breakthrough came when principal research scientist Dr Madhulika Tripathi noticed defects in autophagy in brain samples from preclinical models with elevated blood levels of an amino acid called homocysteine—a known risk factor for stroke.
She wondered if she would observe the same in the liver as well. Sure enough, when homocysteine levels were elevated, the autophagy process in the liver, too, was disrupted. But what really caught her attention was the presence of NASH in these samples.
But how did homocysteine fit into this puzzle involving autophagy and NASH? As the amino acid was known to affect the function of proteins, could this be what interfered with autophagy?
Joined by Assistant Professor Brijesh Singh, the trio probed further, eventually establishing a link between high levels of homocysteine and impaired autophagy. To restore autophagy in the liver, the team hunted for a way to decrease the excessive amounts of homocysteine that were hampering the clean-up process.
After some sleuthing along the body’s biochemical pathways, they discovered that homocysteine could be converted to methionine, another amino acid in the presence of vitamin B12 and folic acid.
By encouraging the conversion into methionine, they could eliminate the harmful effects of too much homocysteine, even though they could not prevent its formation.
“The story just kept surprising us at every turn!” said Madhulika. They had discovered a form of vitamin therapy that could hold the key to reducing levels of homocysteine and delaying the onset of NASH.
They proceeded to confirm their hypothesis by supplementing the diet of preclinical NASH models with vitamin B12 and folic acid. And their intervention worked: it slowed NASH progression and reversed liver inflammation and fibrosis.
Writing the next chapter
The potential use of vitamin therapy in delaying NASH has got everyone in the Yen lab excited.
“Vitamin therapy is affordable, and has little to no side effects. So we’re thrilled about this discovery because we think that maybe this could be a first line treatment for patients,” said Yen. “It may also provide cost savings for Singapore, and could be an alternative offered to people in developing countries.”
But before this can become a reality, the therapy has to be tested in patients with NASH, which is what Brijesh and his collaborator, Associate Professor of Medicine Ayako Suzuki at Duke University will focus on over the next few months after receiving a Duke/Duke-NUS Research Collaboration Award in 2022. They also plan to conduct similar studies in Singapore and the region.
“We have to verify our findings before we can make any clinical recommendations,” stressed Yen.
It is a sentiment that fellow clinicians like Liu and Tan share. They are in the midst of recruiting between 200 to 300 patients for a larger clinical trial to validate their fenofibrate findings. “We hope to develop the best way of delivering fenofibrate, whether it’s in tablet form or by eye drops,” added Tan. Through the trial, the duo will also collect the data needed for regulatory approval of the drug for its new purpose.
For Liu, the potential of fenofibrate extends beyond treating diabetic patients with corneal neuropathy. She hopes to test the drug’s efficacy in treating patients with corneal neuropathy arising from other causes, such as refractive or corneal surgery.
“Currently, there are extremely limited drugs that stimulate nerve regeneration in the eyes. We usually give patients treatments such as steroids or lubricants to relieve their symptoms, but we are not addressing the root problem, which is corneal neuropathy,” she explained.
As for Madhulika and Brijesh, one change they hope to bring about is the emphasis on screening for elevated levels of homocysteine in the clinic. “It is not in the panel of routine tests for patients who suffer from NAFLD. But screening will help identify the cohort of patients who could benefit from vitamin supplementation in future,” said Brijesh.
“Ultimately, our primary motivator is really to do something that can improve the care of patients”
Prof Paul Yen
“Ultimately, our primary motivator is really to do something that can improve the care of patients,” said Yen. “What we hope is that maybe based on our research, doctors would make sure that patients are taking the appropriate tests and treatments.”