The regulatory landscape in Asia is filled with challenges, each of which can be transformed into possibilities and opportunities with the right knowledge and expertise.
The rapid growth of the biomedical and life sciences sectors, population boom, increasing number of clinical trials and expanding biomedical research have meant an unprecedented evolution within the drug and healthcare industry. To balance this scale, the regulatory authorities exert substantial impact on the healthcare landscape through their interaction with key healthcare stakeholders from patients to healthcare manufacturers and providers to ensure continued protection of public health.
Yet, a noticeable vacuum has arisen, making the healthcare environment increasingly complex thereby posing difficulties in succeeding in its mission. A shortage in regulatory expertise, lack of support systems to facilitate regional regulatory policy innovations and diverse regulatory requirements across different countries are just a few issues which present themselves.
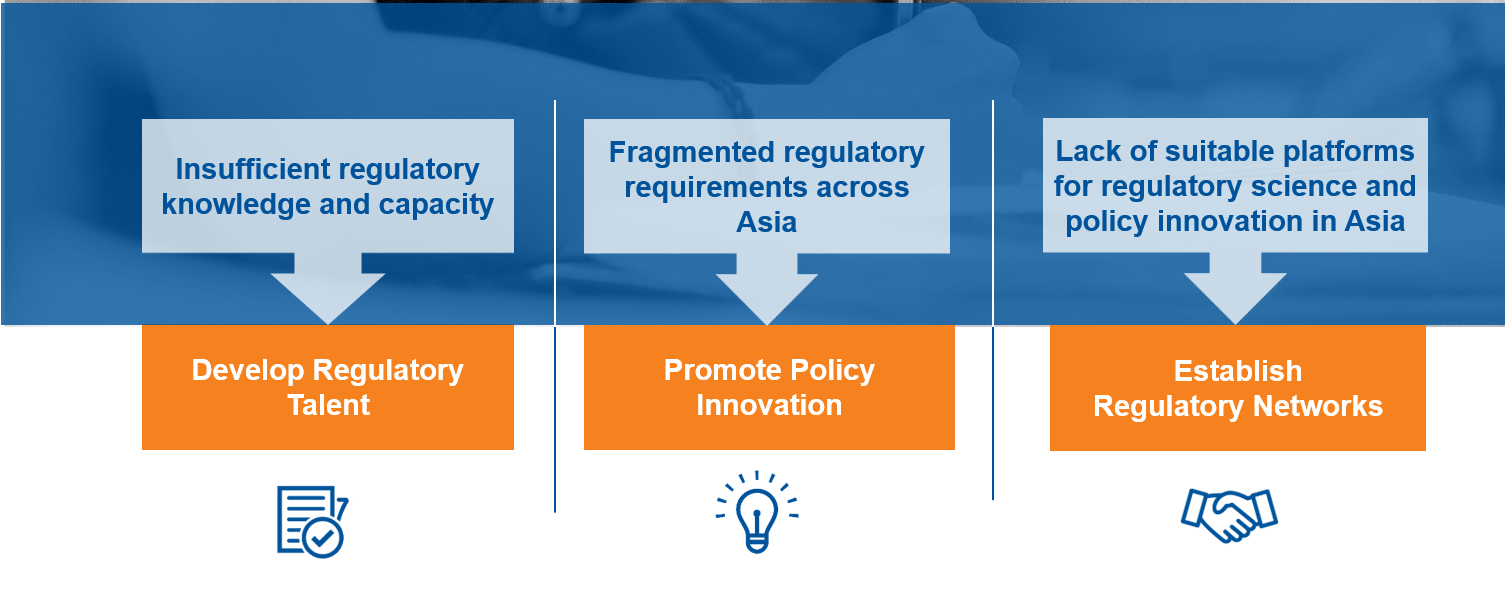
By listening to these unique issues prevalent in Asia, Singapore recognizes a need for the creation of a neutral platform to help regulatory growth take shape and create an environment in which regulatory policy can be developed to create measurable results through improved health outcomes; preventing deaths and illnesses and enhancing the quality of life.In 2014, Singapore launched the first dedicated Centre to promote Regulatory Excellence (CoRE) with the purpose of ensuring patients in Asia have timely access to safe, effective and high quality therapeutic products through regulatory excellence.