 On the first anniversary of the release of the SARS-CoV-2 sequence, the professor in Duke-NUS’ Emerging Infectious Diseases Programme reflects on rallying his lab to sequence SARS-CoV-2 and the need to lay the groundwork now for an even faster start—next time.
On the first anniversary of the release of the SARS-CoV-2 sequence, the professor in Duke-NUS’ Emerging Infectious Diseases Programme reflects on rallying his lab to sequence SARS-CoV-2 and the need to lay the groundwork now for an even faster start—next time.
“I hope they manage to lock that down,” was Professor Gavin Smith’s first thought when news broke in December 2019 that a novel virus was spreading from Wuhan.
“Because if they don’t, it’ll probably become a pandemic.”
But they couldn’t—and it did. The transportation hub was finally closed to the outside world on 23 January 2020. By then, 286 people around the globe were already suffering from—and spreading—the highly infectious novel coronavirus.
And two of these virus carriers, travellers from Wuhan, were already warded in Singapore.
By then, Smith had already become involved. The professor with Duke-NUS’ Emerging Infectious Diseases Programme is a multi-disciplinary researcher who is a renowned expert on respiratory viruses. His team is trained in fieldwork, biosafety level 3 (BSL3) research and the genomic mapping of viruses.
The immediate ask of Smith and his team was to be ready to sequence the novel virus once it hit Singapore’s shores. With that data in hand, they and other scientists could then trace the virus’ journey and identify new mutations over time.
“In the early days, the sequencing data is crucial for contact tracing, so that we can know whether the virus is spreading in the community and monitor how it is adapting to being transmitted by humans,” says Smith, reflecting on how this had all begun.
Comparing the sequences with clinical outcomes would also help to spot mutations that could affect the infectivity, transmissibility and severity of the virus. That’s how his team went on to discover a large deletion in one of the virus’ key genes, called ORF8.
“It was a massive deletion that was quite unexpected,” recalls Smith.
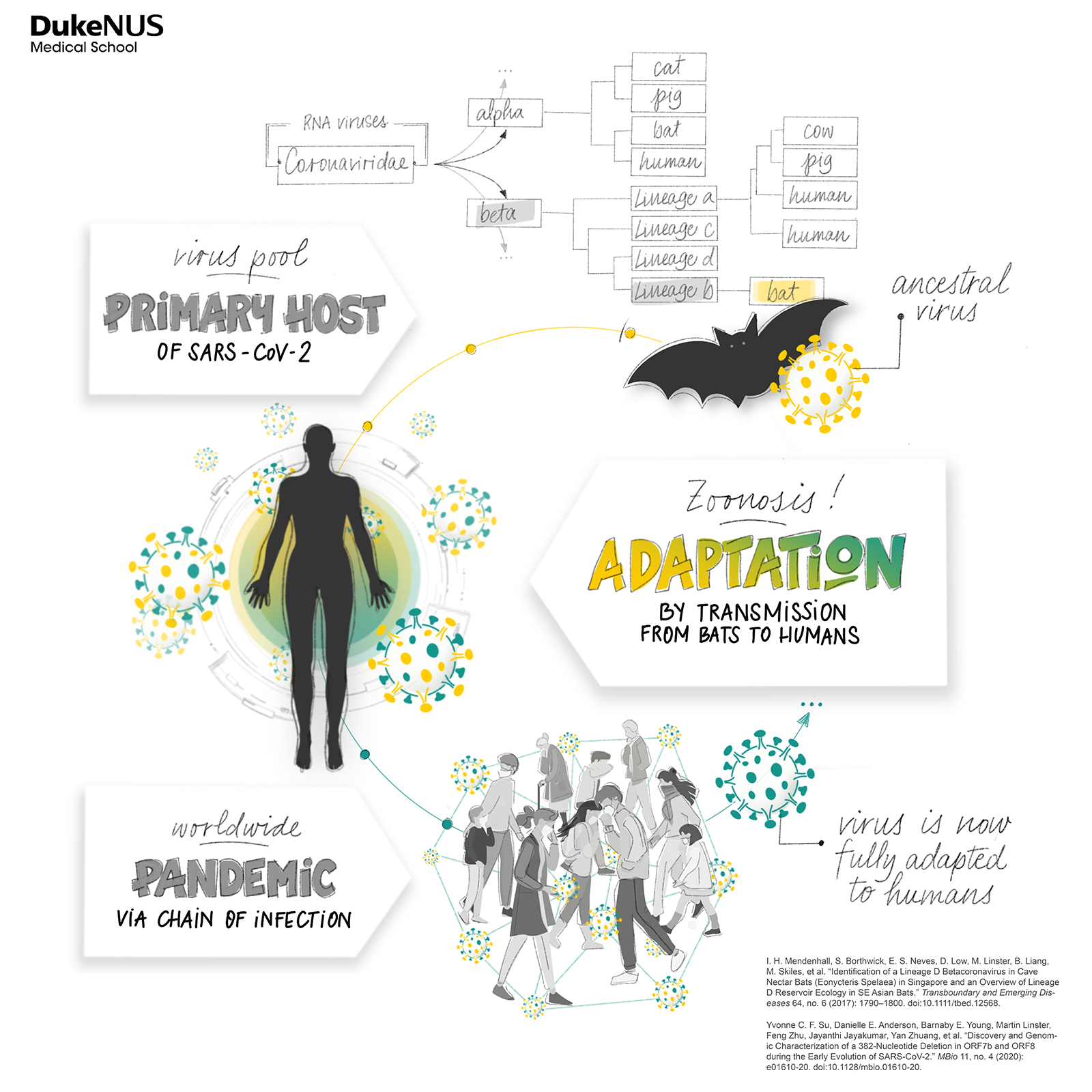
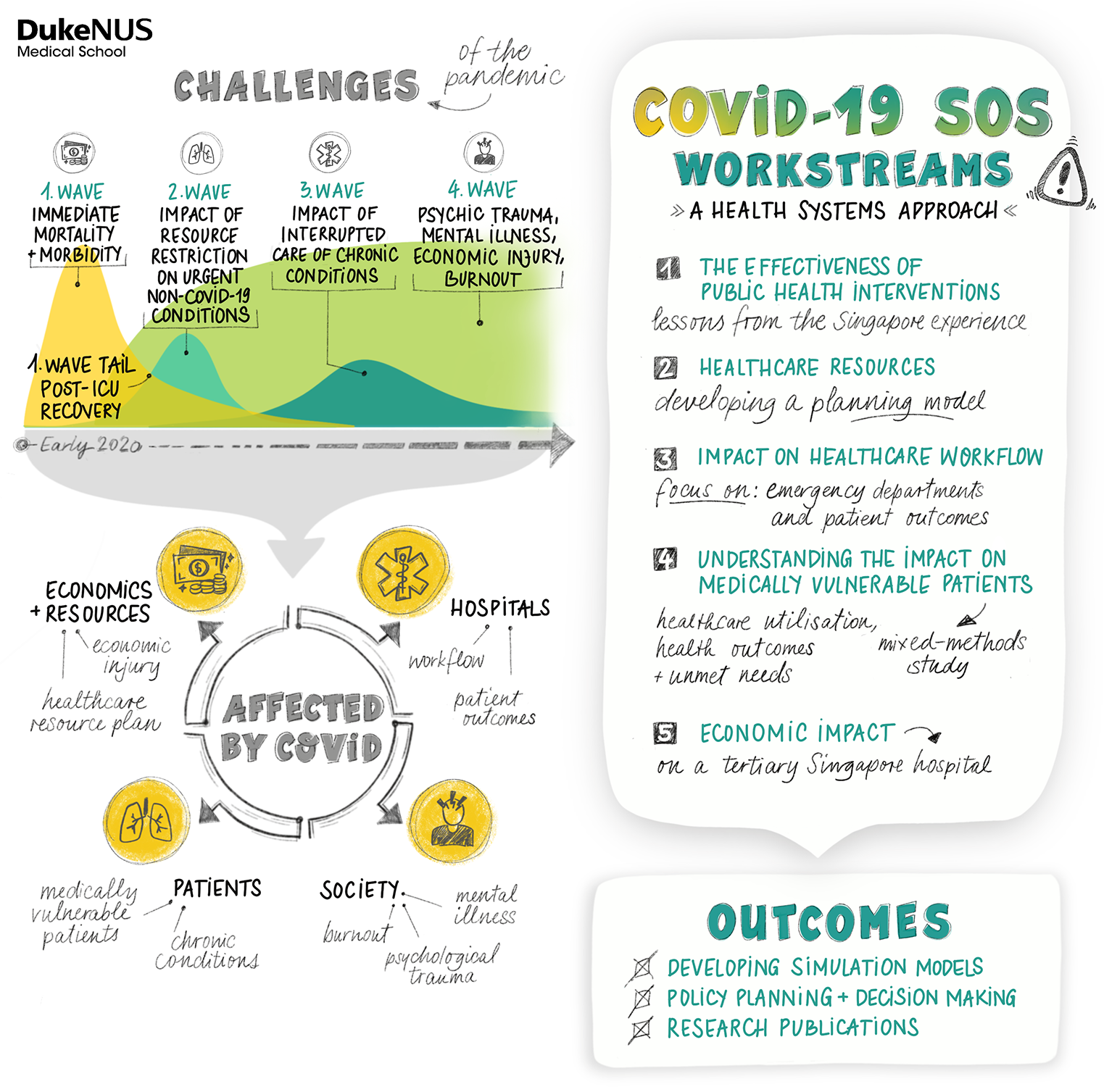
RESEARCH ON THE EVOLUTION OF THE VIRUS, WHICH LED TO DISCOVERIES LIKE THE ORF8 DELECTON BY SMITH AND SU, DEMONSTRATES HOW VIRUSES ADAPT TO THEIR HOSTS THROUGH TRANSMISSION
While Smith’s focus was on the genetic evolution of the virus, sequencing data also underpinned the work of other scientists who used it to develop new tests, better treatments and vaccines.
But all that came later. First, Smith’s team scrutinised the first sequence released to the world of the novel coronavirus, comparing it to existing sequences of similar viruses from animals and humans.
A sequence in sight
Genetic sequencing involves finding out how the four chemical bases in the virus’ RNA—its guanines, cytosines, adenines and uracils—repeat to form a unique chain that can be compared with other chains from the same virus.
“Early on in any pandemic, it’s really useful to have sequencing data,” says Smith. “And the first sequence was released from the Chinese group.”
“We immediately started comparing that sequence with other known coronavirus sequences so that we were prepared for when the first sequences came from patients in Singapore.”
Smith’s lab, reflecting the team’s interests and skills, is set up to sequence respiratory pathogens. Moreover, the team had worked on human coronaviruses—“the regular ones”, says Smith—before SARS-CoV-2.
“Preparation is what we do every day,” says Smith of his team’s mission.
The team travels around the world, working with—and training—partners to conduct robust surveillance of the viruses circulating in animals, particularly bats and small mammals like rats—hotbeds for disease X. They are used to doing their work in protective coveralls. They are accredited N95 mask fitters and used to wearing such masks or even respirators while working. They are experts at handling all kinds of pathogens.
“This kind of research builds capacity,” says Smith. And capacity is precisely what is needed during a pandemic. Ready capacity meant that the team was set to examine the 30,000 bases that make up this novel coronavirus.
“Our focus was on trying to generate the sequencing data as quickly as possible and making sure that we weren’t messing it up,” recalls Smith.
The result—Duke-NUS was the third institution in the world to culture and isolate the SARS-CoV-2 virus, sharing its sequence with the world on 30 January 2020, a mere seven days after the first case of SARS-CoV-2 was detected in Singapore.
“It was an achievement and it shows that we have the capacity to do this work. More importantly, it meant we had the virus and we could start to study it. And it was key for [Professor Wang] Linfa developing the cPass™ that’s become widely used. That was very satisfying.”
Adapting as a family
It was satisfying, too, to be able to reassure his children—seven and eleven at the time—who would bring home the latest talk from the classroom.
“They would come home from school and tell us what they’d heard from their friends,” Smith smiles. “Then we just say ‘Oh, you know that’s not quite what’s going on. This is really what’s happening.”
Together with his wife, Yvonne Su, a then assistant professor with the Emerging Infectious Diseases team, he would reassure them: Yes, this virus is a problem. Yes, it is real. No, you don’t need to be scared.
Were his kids ever scared for their parents? “No, they don’t have any such concept [of what our work involves],” chuckles Smith. “They would forget about it for a while and then a month later, you’d get the same question.”
“We’d say ‘You do know we work on this, right?’”
“And they go ‘Oh? Really?’”
In the months that followed those helter-skelter early days, home-based learning would be added to the mix as the outbreak transformed into a rampant pandemic.
“We were struggling to work out what was going on with the home-based learning, particularly as we were still in the lab every day” says Smith.
“At the same time, we were doing this extra job supporting the national effort to sequence early virus samples that we’d been given, while also still having to do all the regular work and look after the team because everybody was uncertain about what was going on, and it was really stressful.”
Dealing with uncertainty and the joy of new knowledge
By March 2020, it had become clear that the world faced an escalated threat, one that kept on confounding even the highest echelons of public health officials with its unpredictability. A senior World Health Organisation (WHO) official, for example, stated during this time that the asymptomatic transmission of the virus was “very rare”; more senior WHO officials backtracked on the comment the following day.
“What really surprised me was the number of assumptions that were made,” recalls Smith. “That led to definitive statements which were really unhelpful especially at a time when no one really had a clue.”
But the pandemic also brought pleasant surprises. With its mix of bat researchers, immunologists, virologists and vaccinologists, Duke-NUS’ Emerging Infectious Diseases Programme, though small compared with other infectious diseases teams, packed a solid punch.
“In hindsight that was a very effective unit to have. Given how small our department is we made an oversized contribution both nationally and internationally when it comes to the COVID response,” says Smith, who in a year’s time would go on to become the programme’s director.
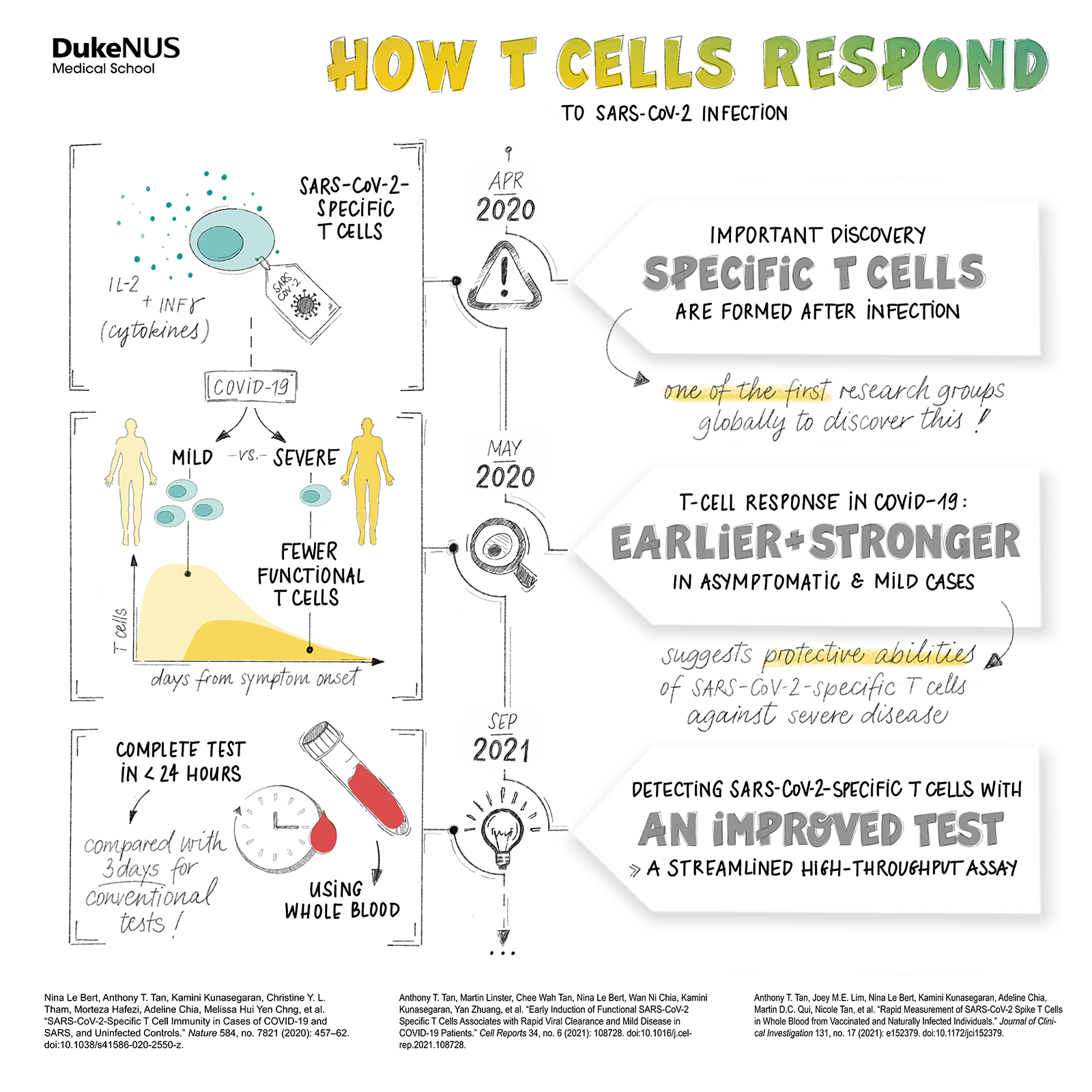
He was especially impressed with the “amazing” research on the immune system’s T cells by immunologist and a fellow professor with the Programme Antonio Bertoletti and how Bertoletti contributed a new understanding of how these cells might counteract the severity of a SARS-CoV-2 infection.
But throughout the heat of the outbreak Smith stuck to one guiding principle—his research should make for a world free from the pandemic.
“Contrary to what some on social media claimed, scientists are overwhelmingly good people,” he shares. “We do this type of stuff because we’re interested in helping.”
“And so, even though people were completely burnt out, they still felt that it’s a duty to try and find solutions.”
The way to the next pandemic
Solutions for Smith meant vaccinations being rolled-out globally and, in future, making Duke-NUS an institution that remained ready to help tackle the next pandemic event.
“The ideal situation is that as immunity builds globally, whether through vaccination or natural infection, the overall severity of the disease becomes less,” he notes.
“It’ll hopefully settle down to a level of severity that’s no greater—but hopefully less— than flu. But even then, it is still a big deal. It is an enormous cost to society."
His crystal-ball gazing would turn out to be spot on. And looking to the next pandemic, Smith muses:
“Pandemics aren’t rare. If you look at the exposure of humans to pathogens in animals, it is enormous.”
While most such exposures or spillovers don’t trigger a pandemic, they happen all the time. And it only takes one virus that happens to be readily transmissible between humans to spark the next outbreak.
With this constant threat, the only response is preparedness.
“We have all sorts of disaster plans right? Fires. Earthquakes and tsunamis. Floods. We now have to have a pandemic preparedness plan that should be updated to match the capacity and skills of the team,” says Smith.
“Before this, the idea that a department or a school should have a pandemic preparedness plan was kind of laughable, right?”
He gestures, already knowing the answer. Yes, it was. Not anymore.















































 The “shadow pandemic” of psychological distress is this clinician-scientist’s top concern.
The “shadow pandemic” of psychological distress is this clinician-scientist’s top concern.
 This Duke-NUS virologist aims to break SARS-CoV-2 infection cycles at a molecular level.
This Duke-NUS virologist aims to break SARS-CoV-2 infection cycles at a molecular level.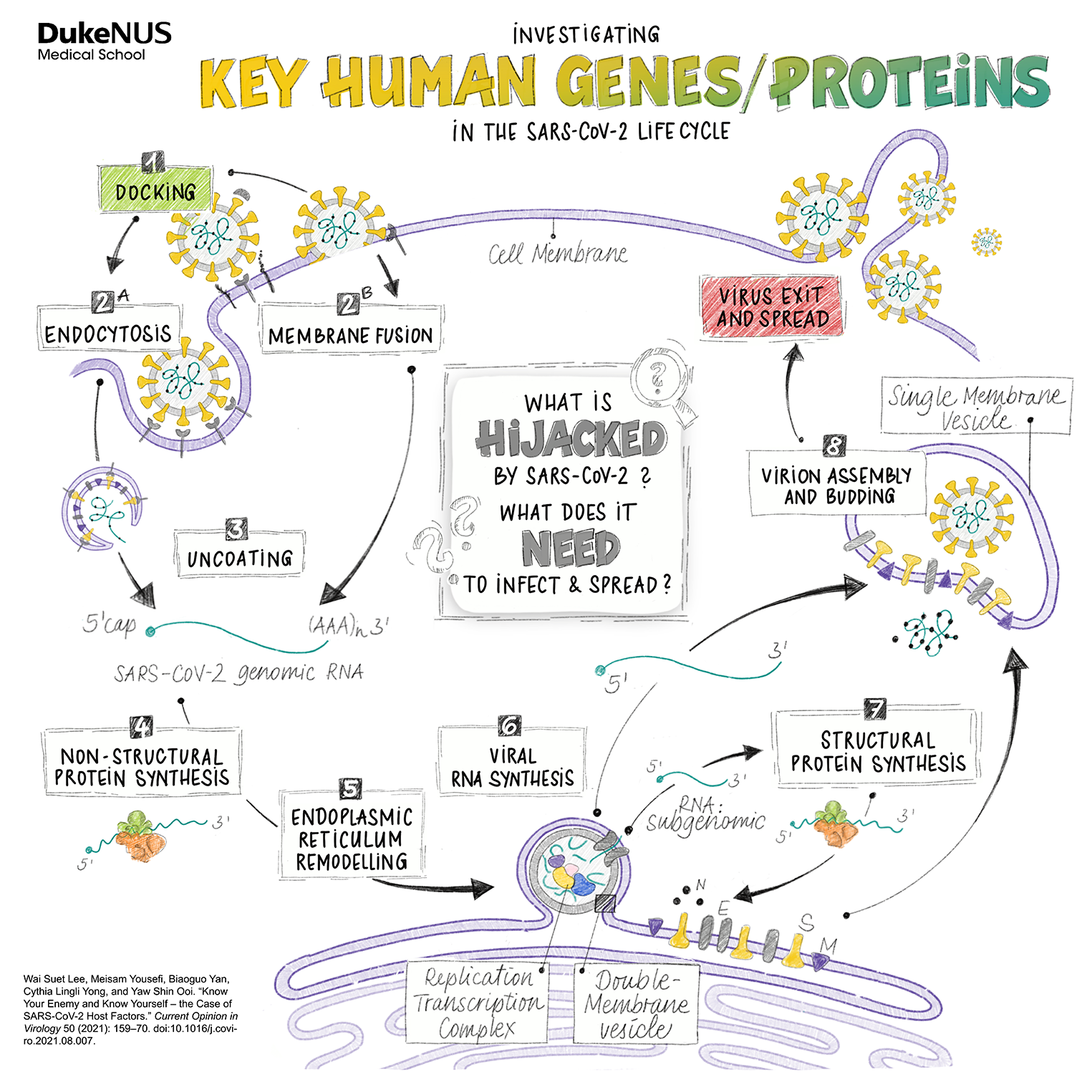
 A Duke-NUS research team chats candidly about their mission to develop a dream vaccine.
A Duke-NUS research team chats candidly about their mission to develop a dream vaccine.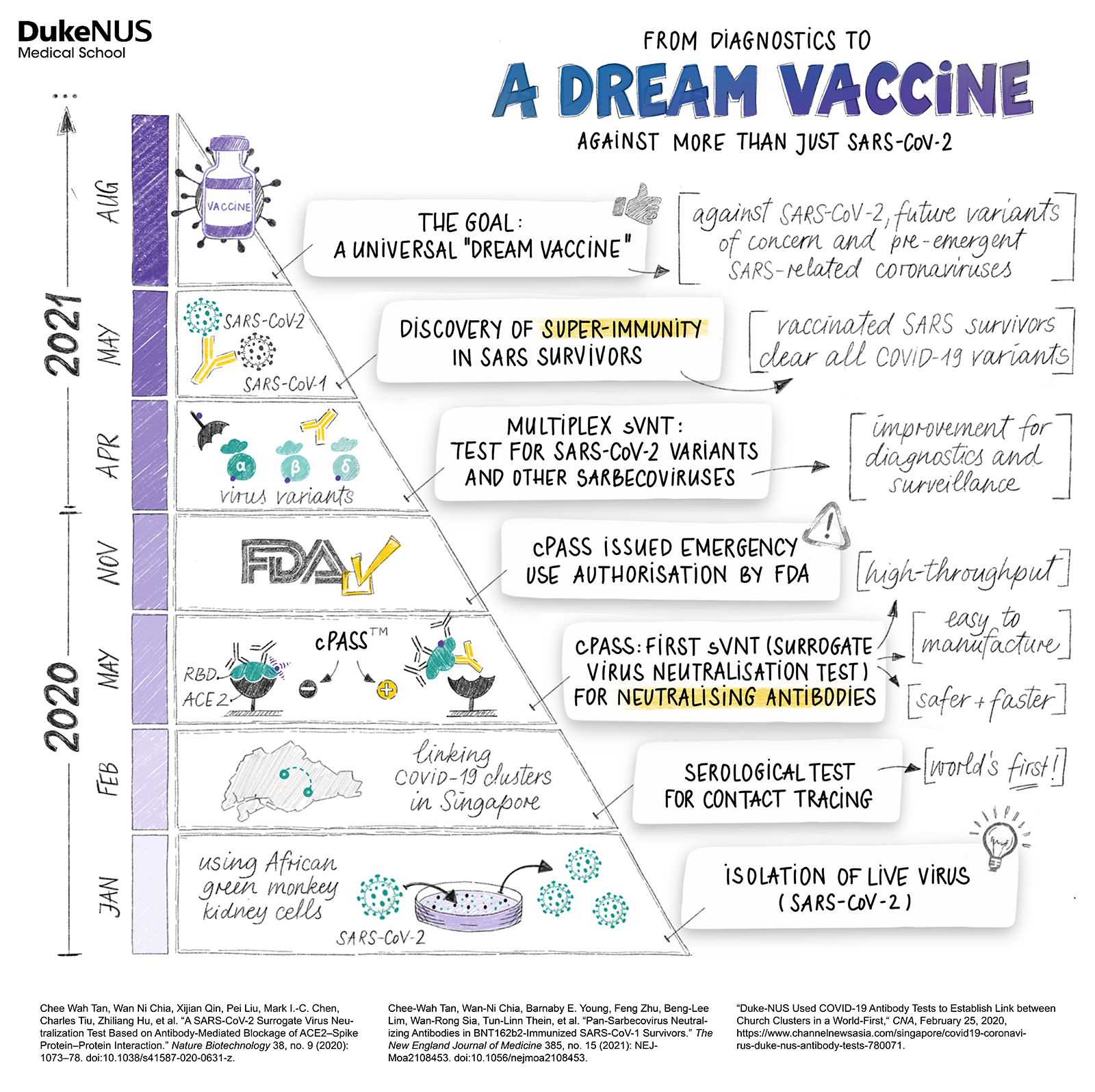
 SARS-CoV-2’s inflammation profile is something quite unique, immunologist
SARS-CoV-2’s inflammation profile is something quite unique, immunologist 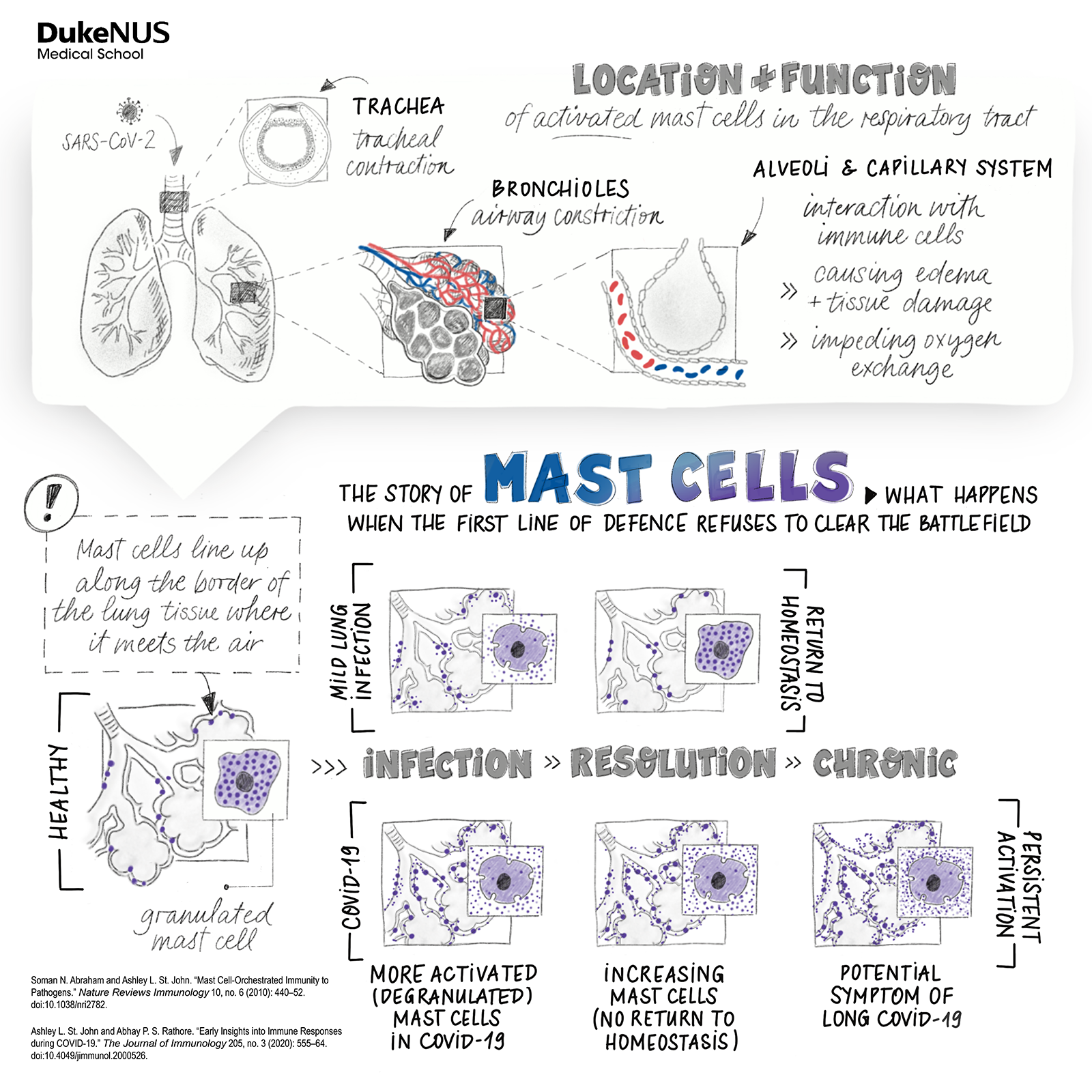
 A clinician-scientist’s journey through the pandemic.
A clinician-scientist’s journey through the pandemic. A data scientist’s calculations help steer the healthcare system through the pandemic.
A data scientist’s calculations help steer the healthcare system through the pandemic.
 This clinician-scientist would rather trade all her accomplishments for a COVID-free 2020.
This clinician-scientist would rather trade all her accomplishments for a COVID-free 2020. Trekking in the Himalayas was when this big Twitter fan first caught wind of a new virus.
Trekking in the Himalayas was when this big Twitter fan first caught wind of a new virus.
 On the first anniversary of the release of the SARS-CoV-2 sequence, the professor in Duke-NUS’ Emerging Infectious Diseases Programme reflects on rallying his lab to sequence SARS-CoV-2 and the need to lay the groundwork now for an even faster start—next time.
On the first anniversary of the release of the SARS-CoV-2 sequence, the professor in Duke-NUS’ Emerging Infectious Diseases Programme reflects on rallying his lab to sequence SARS-CoV-2 and the need to lay the groundwork now for an even faster start—next time.
 Studying the information conveyed in genes, this scientist recalls the intense days of the pandemic.
Studying the information conveyed in genes, this scientist recalls the intense days of the pandemic. The need for a self-sufficient Singapore motivates one researcher’s quest for a SARS-CoV-2 vaccine.
The need for a self-sufficient Singapore motivates one researcher’s quest for a SARS-CoV-2 vaccine.
 “SARS is gone and it won’t be coming back, so this is a waste of money,” immunologist
“SARS is gone and it won’t be coming back, so this is a waste of money,” immunologist  From Hendra to SARS-CoV-1, Wang Linfa brought all this experience to bear in the fight against COVID-19.
From Hendra to SARS-CoV-1, Wang Linfa brought all this experience to bear in the fight against COVID-19.


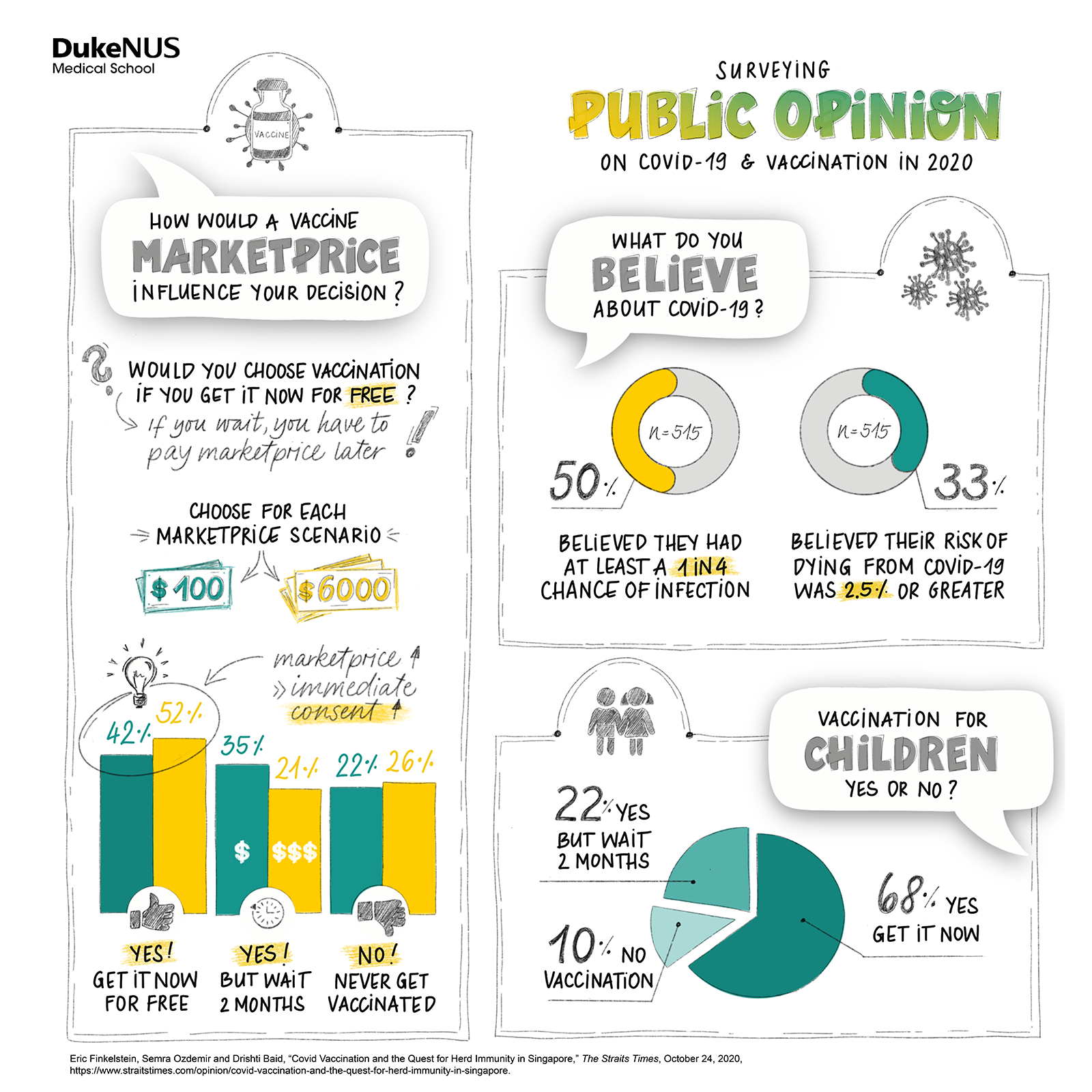
 How did COVID-19 affect Singapore’s national mood?
How did COVID-19 affect Singapore’s national mood? Leveraging the pandemic, this surgeon-innovator proves that even in healthcare, new ideas can be turned into innovations fast.
Leveraging the pandemic, this surgeon-innovator proves that even in healthcare, new ideas can be turned into innovations fast.


 This evolutionary biologist-turned-virus-decoder finds that a quarter of Singapore’s diseases stem from a virus variant, and that her daughter helped a classmate through a difficult COVID situation.
This evolutionary biologist-turned-virus-decoder finds that a quarter of Singapore’s diseases stem from a virus variant, and that her daughter helped a classmate through a difficult COVID situation. This authority on T cells pivots to lend his expertise to tackle the new threat.
This authority on T cells pivots to lend his expertise to tackle the new threat.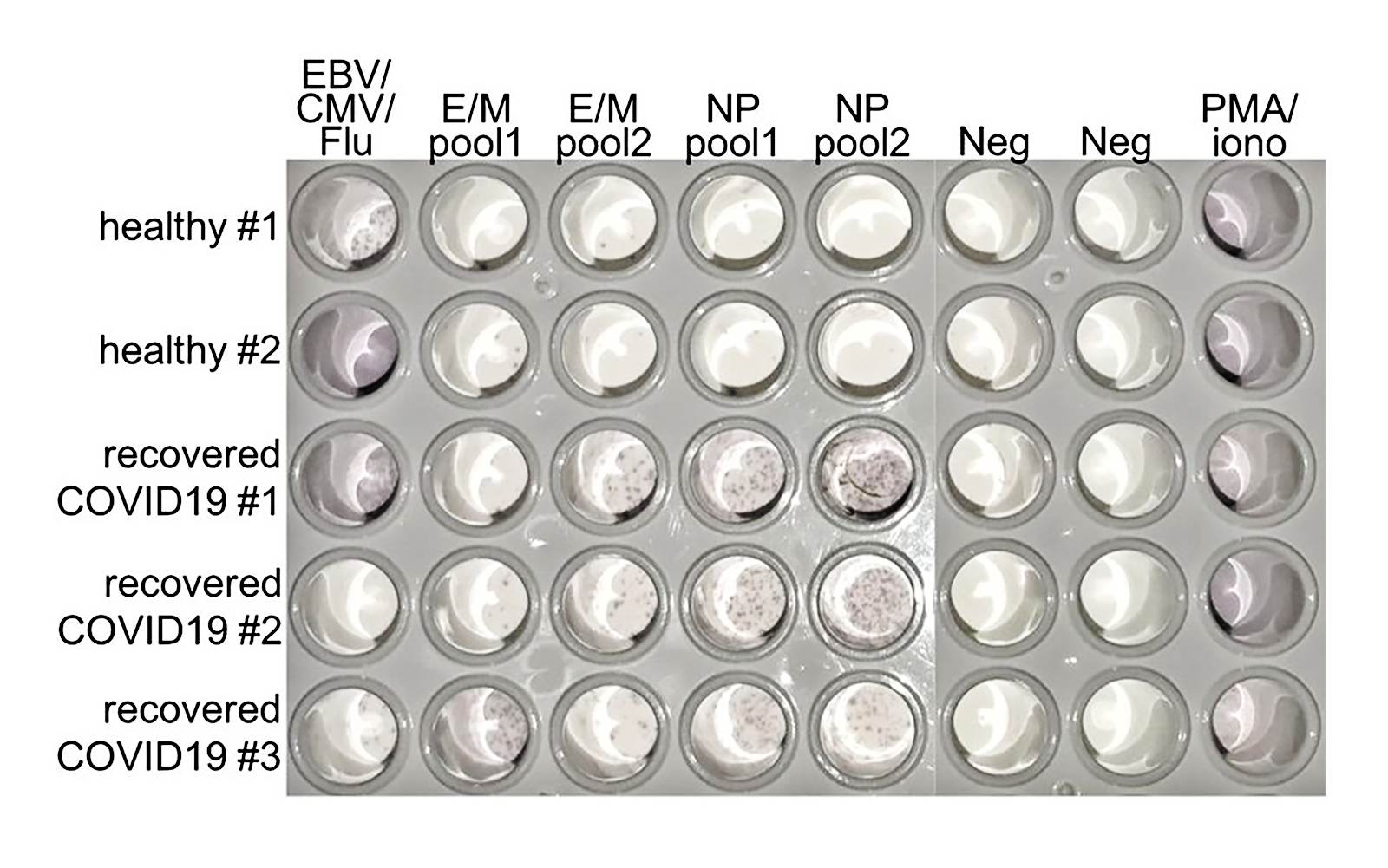

 This immunologist turned a torrent of data about the early immune responses of SARS-CoV-2 patients worldwide into an accessible data stream.
This immunologist turned a torrent of data about the early immune responses of SARS-CoV-2 patients worldwide into an accessible data stream.
 Duke-NUS’s education team rallies for a second time to deliver clinical exams at the height of the Circuit Breaker.
Duke-NUS’s education team rallies for a second time to deliver clinical exams at the height of the Circuit Breaker.
 The Bertoletti lab would work flat out through the Circuit Breaker, digging beyond antibodies to discover more about the immune system’s response to SARS-CoV-2.
The Bertoletti lab would work flat out through the Circuit Breaker, digging beyond antibodies to discover more about the immune system’s response to SARS-CoV-2.

 Chia Wan Ni and Tan Chee Wah, two early career researchers in the “Linfa lab”, achieve a world-first in serological testing and develop a SARS-CoV-2 test.
Chia Wan Ni and Tan Chee Wah, two early career researchers in the “Linfa lab”, achieve a world-first in serological testing and develop a SARS-CoV-2 test.
 An educator adapts to restrictions and being in a new job while alone in a new country.
An educator adapts to restrictions and being in a new job while alone in a new country.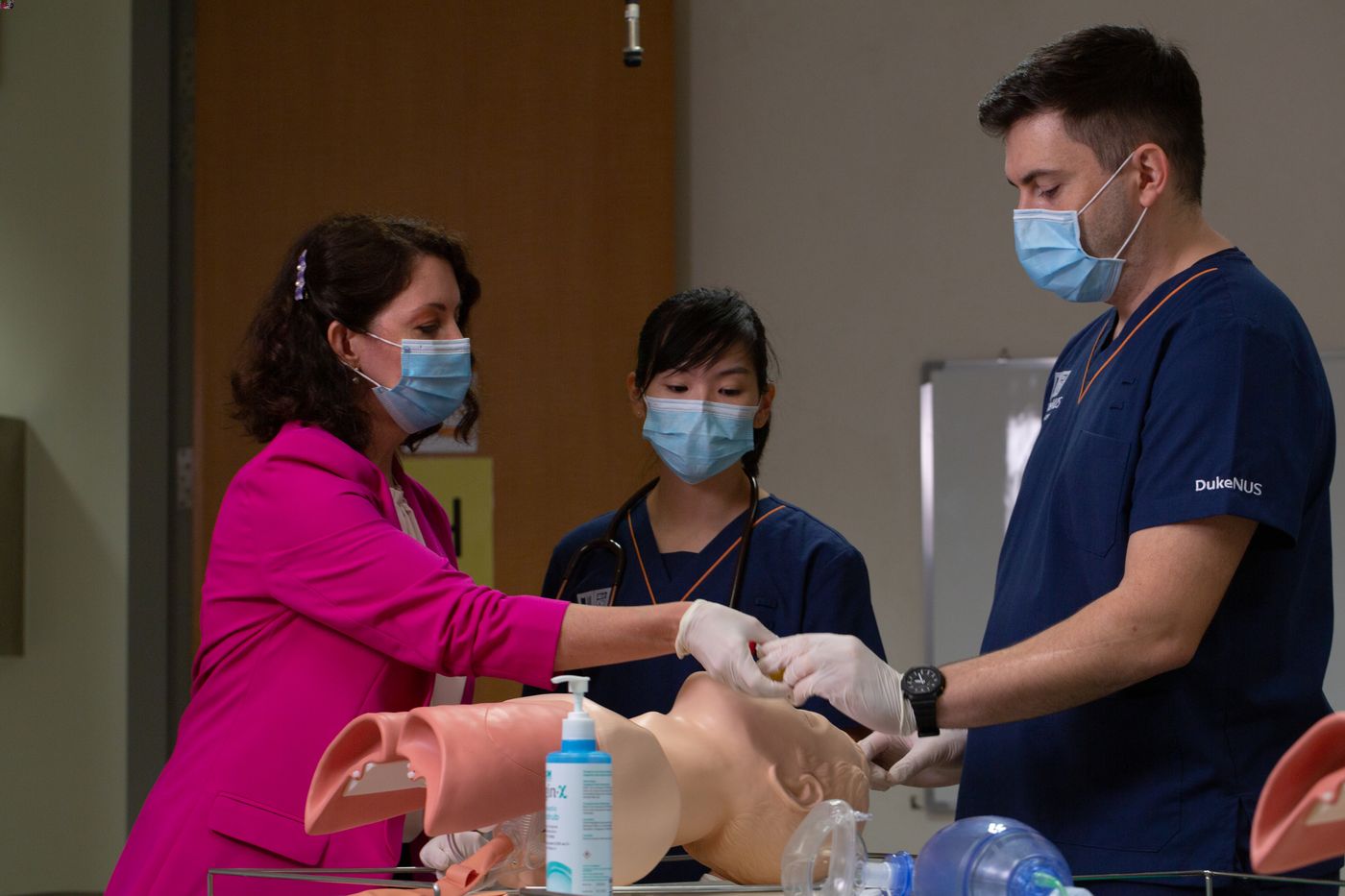
 It was Friday lunchtime when the news came that clinical training across Singapore’s healthcare system was suspended with immediate effect.
It was Friday lunchtime when the news came that clinical training across Singapore’s healthcare system was suspended with immediate effect. A veteran of high-containment labs returns to the hot zone just in time to sequence SARS-CoV-2.
A veteran of high-containment labs returns to the hot zone just in time to sequence SARS-CoV-2.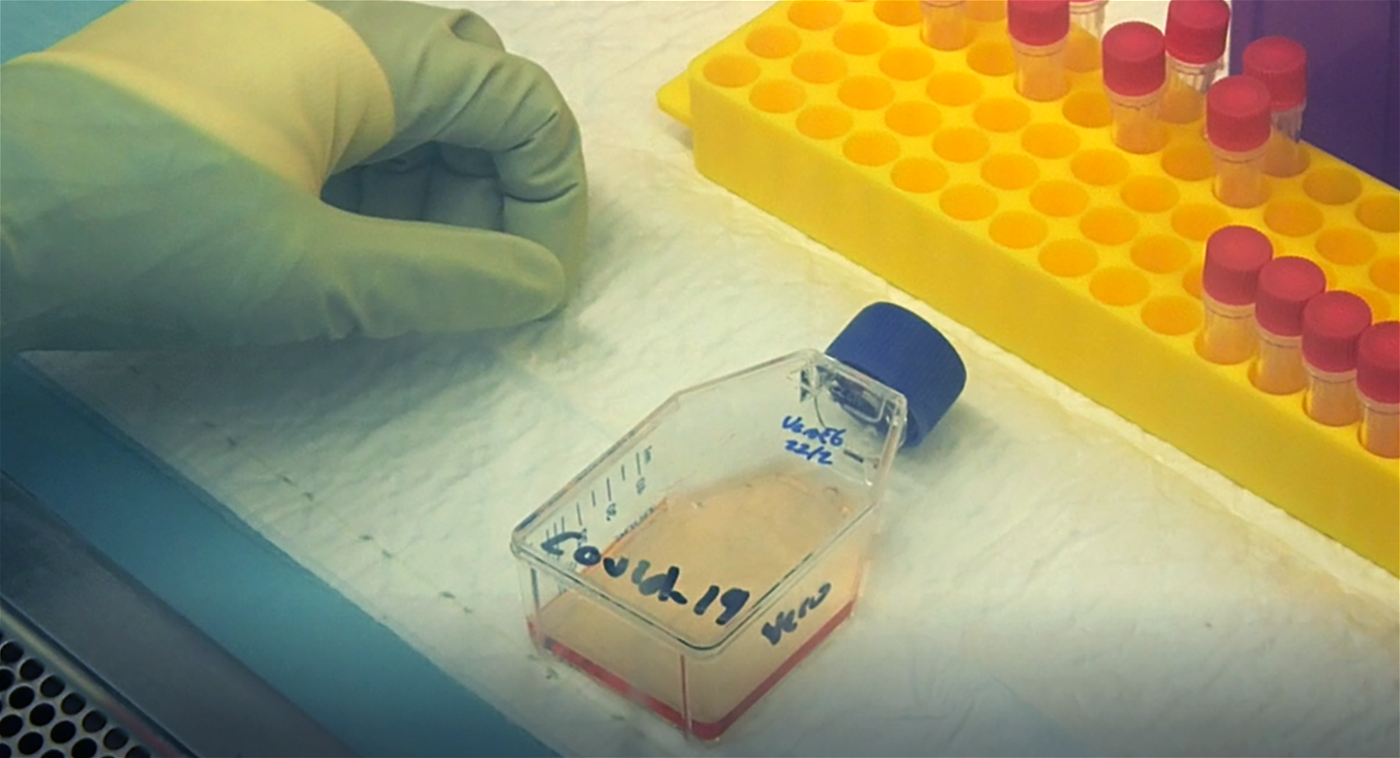 A MEMBEr OF THE DUKE-NUS TEAM WORKS IN THE BSL3 LAB ON ISOLATING THE NOVEL CORONAVIRUS
A MEMBEr OF THE DUKE-NUS TEAM WORKS IN THE BSL3 LAB ON ISOLATING THE NOVEL CORONAVIRUS
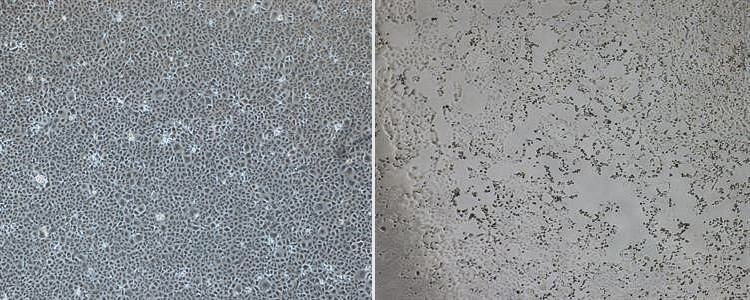
 The morning after the first case was reported in Singapore, about 10 experts from Duke-NUS’ safety team and
The morning after the first case was reported in Singapore, about 10 experts from Duke-NUS’ safety team and  In the SARS-CoV-2 pandemic’s city of origin, a Duke-NUS scientist experiences first-hand an unprecedented level of medical security.
In the SARS-CoV-2 pandemic’s city of origin, a Duke-NUS scientist experiences first-hand an unprecedented level of medical security.


