In national health systems, the regulation of health products, health services and health professionals are traditionally treated as distinct domains with the regulatory frameworks managed by different regulatory departments or agencies. While there may be some degree of consultation and coordination, they largely operate independently from each other on a day-to-day basis.
The Duke-NUS Centre of Regulatory Excellence (CoRE), which is celebrating its tenth anniversary in 2024, was initially established with a primary focus on health products regulation (1). In CoRE’s initial years, there was some consideration of health services regulation when issues related to medical technology and combination products regulation were being studied. But the predominant focus of our educational, think-tank and advisory activities was on health products and regulatory systems strengthening for pharmaceuticals and medical devices. However, in recent years, the scope of the Centre has broadened to include think-tank and research projects in health services regulation, in partnership with Singapore’s Ministry of Health.
With the expansion of the Centre’s scope, the converging of health products and health services regulatory issues has become increasingly evident as the Centre has studied global trends and issues related to the regulation of artificial intelligence (AI) and digital health, advanced therapies, precision medicine and public health, and patient engagement. During the COVID-19 pandemic, it also became clear that there was a critical need in national health systems operating under immense pressure for much tighter coordination across health products and services regulation to ensure early approvals of and access to safe, efficacious, and quality therapeutics, diagnostics, and vaccines.
Figure 1 illustrates the health domains where key health regulatory frameworks are converging, based on CoRE’s observations and experience. Global health regulatory policy is considered due to the increasing interconnectivity and partnership among international regulators as well as the Centre’s collaboration with the SingHealth Duke-NUS Global Health Institute. However, the regulation of health professionals is not included as the Centre’s scope does not currently cover this complex and challenging area.
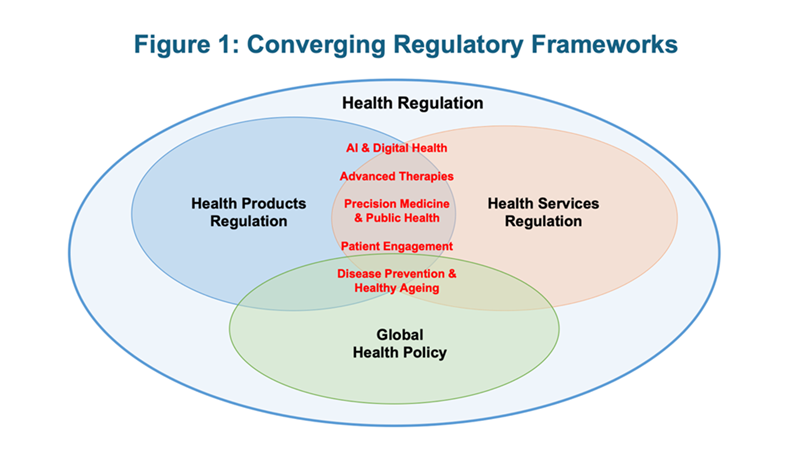
To optimise innovation and equitable access in and across these domains, policy makers, regulators, and other stakeholders in the broader health ecosystem - including industry, patients and academics - need to work together to enhance and align relevant regulatory frameworks. This is especially so for regulation that is still nascent and needs further elucidation or development. A prime example is the regulation of AI and digital health. Without adequately defined digital strategies and regulatory frameworks for generative AI in health that address digital health concerns such as data security and interoperability, the resulting lack of governance and public trust will hinder the ability of governments and health systems to harness the full benefits of AI and digital health (2). In addition, these technologies raise ethical issues that affect regulation but which have not yet been fully thought through in the contexts of different societies and cultures.
Given that these concerns span the delivery of services and use of products, studying the intentional integration of regulatory frameworks for health products and health services should be encouraged. A commitment to converging health products and health services regulation will engender important multi-sectoral perspectives. Convening stakeholders within and beyond the health sector to collaboratively develop appropriate integrated regulatory frameworks will support the ecosystem for health products and health services innovations that are safe, relevant and impactful. This will not only strengthen health systems but also better serve future health needs of patients and populations, thereby enhancing overall population health and quality of life.
1. Regulating AI and Digital Health
Digital health encompasses a multitude of complex health technologies with applications throughout the entire healthcare delivery chain. These include Software as Medical Devices (SaMD), digital therapeutics, wearables, telehealth and telemedicine. While existing frameworks have been largely developed by health product regulators on SaMD, the impact and implications of deploying these technologies may intertwine with telemedicine which falls under the oversight of health services.
The impact of AI and machine learning on healthcare is far reaching. In spite of the immense potential for positively transforming healthcare, many unresolved concerns that AI has prompted in all sectors of society - beyond just the health sector - has resulted in an increasing demand for stronger governance and regulatory guidelines.
Diverse legislation and guidelines have been rolled out globally. These range from the European Union’s ambitiously prescriptive Artificial Intelligence Act to more technically oriented guidelines on SaMD issued by various national regulatory agencies (3,4). Regulatory sandboxes for policy makers and industry have also been set up to provide safe harbour settings for testing appropriate regulatory approaches for developing technologies. One example was the Singapore Ministry of Health’s Licensing Experimentation and Adaptation Programme (LEAP) for telemedicine (TM) that ran from 2018 to 2021 to better understand the risks and co-create risk mitigation measures with industry prior to licensing of TM service providers under the new Healthcare Services Act (HCSA) from 2023 (5).
The fundamental challenge is that the speed and scope of digital health development tend to quickly outpace the conventional incremental way in which legislation and regulations are formulated. Rapid advancements in generative AI such as ChatGPT have sparked calls by governments, policy makers and researchers to halt further development and first address fundamental issues of safety, interoperability, data sharing, data security and ethics (6,7). To help progress the global conversation in this nascent field, there has been a flurry of international and national initiatives. The World Health Organization recently issued a guidance discussing the application, challenges, ethics and governance of large multi-modal models (LMM) in healthcare and medicines (8). Recognising the need to engage expertise from the wider ecosystem, Singapore has partnered with tech companies and industries across different sectors to form the AI Verify Foundation to promote responsible use of AI. The island city state is also seeking inputs from the international community on a new Model AI Governance Framework for Generative AI that expands an existing Singapore framework from 2019 that only covers traditional AI to include Generative AI by mid-2024 (9,10).
Given the diverse applications of AI and digital health across the healthcare sector and the challenge of formulating fit-for-purpose and trustworthy regulatory frameworks, an overarching approach that more holistically addresses AI and digital health governance under integrated health services-health products regulatory frameworks could help yield clearer outcomes as policy makers, regulators, developers, healthcare professionals and patients engage collaboratively.
2. Regulating Advanced Therapies
Advanced therapies are another example of complex therapies with regulatory requirements significantly different from pharmaceutical and biological products. Targeted cell and gene therapies hold great promise but pose regulatory and reimbursement challenges that limit their access in developed but especially in low-and-middle income countries. Often, the clinical designs are small, open-label and non-randomised single-arms studies (11). These products also require complex manufacturing processes, often in small batches in specialised laboratories. These therapies are also still extremely expensive, thus raising health financing, subsidy and equitable access concerns. By 2023, 24 Advanced Therapy Medicinal Products (ATMPs) had been approved in the European Union, while 34 cellular and gene therapy products had been licensed by the US Food and Drug Administration (FDA) (12,13). In Singapore, five Class 2 Cell, Tissue or Gene Therapy Products had been registered as of January 2024 and regulated under the Health Products (Cell, Tissue and Gene Therapy Products 2021) (14,15). Within Singapore’s cell therapy ecosystem, there is a myriad of multi-sectoral stakeholders including scientific, clinical, manufacturing, regulatory and academic partners across the entire product lifecycle. Currently, good manufacturing practices and other regulatory guidances fall under health products regulation, while the use and administration of these advanced therapies comes under the ambit of health services regulation (16). To meet the increasing demand for clinical use of cell therapies, the Advanced Cell Therapy and Research institute (ACTRIS) was set up in April 2020 as a business unit of the Consortium for Clinical Research and Innovation, Singapore (CRIS) to be the national and regional Centre of Excellence for discovery, process development and manufacturing of cellular-based therapeutics (17). Applying a more integrated health services-health products regulatory framework that also includes health technology assessment (HTA) considerations and convening the various stakeholders to consider holistic solutions would help to address the challenges in discovery, process development, manufacturing, and clinical use more effectively and efficiently.
3. Regulating Precision Medicine and Precision Public Health
With decreasing costs of genomic sequencing and increasing computational power, large-scale cohort studies using huge datasets have been initiated to study inherited disease causation and progression as well as human variations in response to medicine at a population level. One such consortium, the International Hundred Thousand Plus Consort Consortium, reported 103 large-scale cohorts with an overall estimate of 50 million participants across 43 countries (18). Genomic, phenotypic and lifestyle data collected in such precision health initiatives can be linked to electronic health records and allow a more precise data and value-driven approach to improve patient outcomes.
Singapore’s 10-year National Precision Medicine strategy is coordinated by Precision Health Research, Singapore (PRECISE), also a business unit of CRIS. Phase 1 of this national initiative established a Singapore reference database of 10,000 genomes and Phase 2, launched in 2020, aims to generate the genomes of 100,000 healthy Singaporeans and 50,000 people with specific diseases. Clinical implementation of precision medicine approaches will be piloted and the data infrastructure established for linkage of genomic data with electronic health records and other data types (19).
Ultimately, precision medicine is envisaged to be implemented on a large scale in Singapore and linked to phenotypic data in population databases and medical records to provide deeper insights into risk identification and prevention of diseases and adverse drug reactions. This will offer an effective means of translating research into more efficient healthcare delivery systems and better health for Singaporeans. A successful example was the implementation of genetic testing of HLA-B*1502 prior to the use of carbamazepine in patients with Asian ancestry in Singapore and its tailored approach to different at-risk alleles (20,21).
Given the need to ensure data privacy and governance while actively promoting translational research, it is again obvious that an integrated health services-health products regulatory framework could provide a more seamless approach to help bridge science, policy and practice issues required for the effective translation of genomics in public health.
4. Regulating with Patient Engagement
The concept of patients as partners in healthcare first gained prominence during the early years of the HIV/AIDS epidemic in the US in the 1980’s (22). Since then, regulators have increasingly recognised that patients have a key role in the development, regulation and safe use of medicines and major regulators like the US FDA, the European Medicines Agency (EMA) and Japan’s Pharmaceutical & Medical Devices Agency (PMDA) have systematically included patient engagement as part of their regulatory processes. However, as was determined during roundtables organised by CoRE from 2019 to 2021 (23), engagement with patients by regulators and health policy makers in Asia and the Pacific is still relatively nascent.
One of the key lessons from the COVID-19 pandemic was the importance of public trust in science. Vaccine misinformation had and continues to have significant adverse impact on national vaccination uptake and public health. The post-pandemic period is an opportune time to promote collaboration of regulators and patients in improving regulatory decision making (24). Patient lived experience could be included throughout the entire life cycle of health services and products including product development, regulatory decision making, financial reimbursement, and the appropriate use of health products in health systems (25). In healthcare institutions, systematic patient engagement helps to improve the patient experience. At the wider health system level, integrating health products and health services regulation would support the establishment of neutral multi-stakeholder platforms for sustained patient engagement in the health ecosystem.
5. Regulating for Disease Prevention and Healthy Ageing
At the core of public health lies the principle of prevention. Regulatory measures play a pivotal role in promoting health and mitigating disease risks. This underscores the significance of proactive, appropriate and effective regulation to safeguard public health. In this regard, exploring a more integrated approach to assess the impact of health products and health services regulation in the context of public health and global health policy is a field that has not drawn much attention to date but holds great promise for strengthening health systems.
For example, the strategies for effectively regulating public health interventions such as vaccination programmes in ways that mitigate misinformation and promote uptake need to be further studied across the health products and health services continuum. The indispensable role of regulation in addressing and managing emerging health threats is well recognised but from the experience of the COVID-19 pandemic, also requires further review of ways to proactively coordinate health products and health services regulation to strengthen health systems for better management of future public health emergencies.
The overarching area of significance is how to enhance health regulatory frameworks, shape translational research directions and implement public health measures to better support rapidly ageing populations for longevity and healthy living. Singapore is projected to become a “super-aged” society in 2026 where the population proportion of individuals aged 65 years and older is about 21 per cent (26). To address the needs for better health and quality of life, preventive health has become the main thrust for Singapore’s Ministry of Health, anchored by a flagship programme known as “Healthier SG” (27). This national initiative promotes personalised health plans and encourages Singaporeans to take proactive steps to manage their health, prevent the onset of chronic diseases and have strong support to lead healthier lifestyles. This involves individuals being linked to a preferred one-stop family clinic for health promotion, prevention and care. The programme also encompasses financial subsidies for medications, consultations and laboratory tests that may be provided for patients with chronic conditions under certain conditions.
Singapore was recently categorised as a “Blue Zone 2.0” by Dan Buettner who popularised the concept of Blue Zones, which are regions in the world where people apparently live longer than average and have more centenarians (28). This was based on the demographic work of Michel Poulain and colleagues in 2004 (29), and the original examples included Okinawa Prefecture in Japan, Sardinia in Italy and Icaria in Greece where longevity was attributed to their culture and lifestyle (30,31). Singapore defies this initial concept and has been described as an “engineered” Blue Zone, with local health experts suggesting that Singapore could even advance to the next phase of Blue Zone 3.0 (32). What is also relevant for Singapore in the context of Healthier SG is the promotion of healthy ageing by drawing on the latest scientific research for early life interventions rather than waiting to treat diseases when they have already manifested (33). This could help to better prevent or manage cardiovascular disease, cancer and Alzheimer’s disease.
While these ideas need further study and discussion, it should be immediately obvious that the proactive integration and converging of heath products and health services regulation are required to follow through on studies, research and implementation of these concepts and approaches for evidence-based healthy ageing. These would also involve the earlier mentioned areas of AI and digital health, advanced therapies, precision medicine and patient engagement.
Regulating the Future of Health
This perspective summarises the key areas that define the theme of "Regulating the Future of Health - Converging Products & Services Regulation for Access, Innovation & Sustainability” for CoRE’s tenth anniversary year and which will also drive key priorities for the centre’s next phase. CoRE’s 10th Anniversary Conference to be held in October 2024 will adopt this theme and convene experts, thought leaders, policymakers, and stakeholders from around the world to engage in discussions concerning the evolving landscape of health regulation. With a focus on the three pivotal areas of Regulating AI, Regulating Digital Health & Precision Medicine, and Regulating for Disease Prevention and Healthy Ageing, the Conference aims to chart a course towards a future where innovation thrives, access to healthcare is equitable, and public health is safeguarded through effective and holistic health regulation.