Back
Tuesday, 21 Jul, 2020
Arcturus Therapeutics–Duke-NUS clinical trials for COVID-19 vaccine candidate approved to proceed
- Human dosing of LUNAR-COV19 expected soon.
- Differentiated STARR™ mRNA vaccine expected to produce humoral and cellular immunity at very low doses.
- New preclinical data demonstrates neutralising antibody titres continue to increase for 50 days after a single administration.
San Diego/Singapore, 21 July 2020 – Arcturus Therapeutics Holdings Inc. (the “Company”, “Arcturus”, Nasdaq: ARCT), a leading clinical-stage messenger RNA medicines company focused on the discovery, development and commercialization of therapeutics for rare diseases and vaccines, and Duke-NUS Medical School, Singapore’s flagship research-intensive graduate entry medical school, today announced that the Clinical Trial Application for COVID-19 vaccine candidate LUNAR-COV19 has been approved to proceed by the Singapore Health Sciences Authority. Arcturus and Duke-NUS partnered to develop a coronavirus vaccine using Arcturus’ STARR™ technology and a unique platform developed at Duke-NUS allowing rapid screening of vaccines for potential effectiveness and safety.
Arcturus and Duke-NUS will initiate human dosing of LUNAR-COV19 as soon as possible. The healthy volunteer study will evaluate several dose levels of LUNAR-COV19 in up to 108 adults, including older adults. Follow-up will be conducted to evaluate safety, tolerability and the extent and duration of the humoral and cellular immune response.
“The approval of the Clinical Trial Application for LUNAR-COV19 is a critical milestone for Arcturus. We are excited to advance this promising vaccine candidate into clinical trials. Based on our preclinical data, we believe that our self-replicating mRNA-based approach may produce high rates of seroconversion and robust T-cell induction with a potential single administration, at very low doses. The LUNAR-COV19 profile is meaningfully differentiated and may facilitate the mass vaccine campaigns necessary to target hundreds of millions of individuals globally,” said Joseph Payne, President and CEO of Arcturus.
Professor Ooi Eng Eong, Deputy Director of the Emerging Infectious Diseases Program at Duke-NUS, said, “Preclinical studies on LUNAR-COV19 have shown very promising findings, including the possibility that a single dose of this vaccine may be sufficient to trigger robust and durable immune responses against SARS-CoV-2. We are very eager to start the first-in-human clinical trial here in Singapore and advance LUNAR-COV19 on its journey to becoming a potential commercial vaccine.”
“There is a tremendous global imperative to develop effective preventive measures for COVID-19 infections. We are heartened by the rapid and promising progress in our vaccine collaboration with Arcturus as we move forward into clinical trials,” said Professor Thomas M. Coffman, Dean of Duke-NUS Medical School.
The STARR™ Technology platform employed in LUNAR-COV19 combines self-replicating mRNA with LUNAR®, a proprietary nanoparticle delivery system optimized for mRNA molecules. The efficiency and self-replicating nature of the approach were designed to enable very low doses, and a potential single vaccine administration. Prior animal data has demonstrated robust humoral and cellular immunity elicited at doses as low as 0.2 µg of LUNAR-COV19. Additionally, Arcturus demonstrated 100% seroconversion for anti-SARS-CoV-2 neutralizing antibodies with a very low single dose (2.0 µg).
New preclinical data demonstrate that neutralising antibody levels in response to a single administration of LUNAR-COV19 (0.2, 2.0, 10.0 µg) continue to increase over 50 days. The increasing antibody levels are attributed to the self-replicating mRNA of LUNAR-COV19. These results were obtained using a Luminex bead assay. A 1/2000 serum dilution was assayed for neutralizing IgG antibodies in the mouse serum every 10 days for 60 days post vaccination.
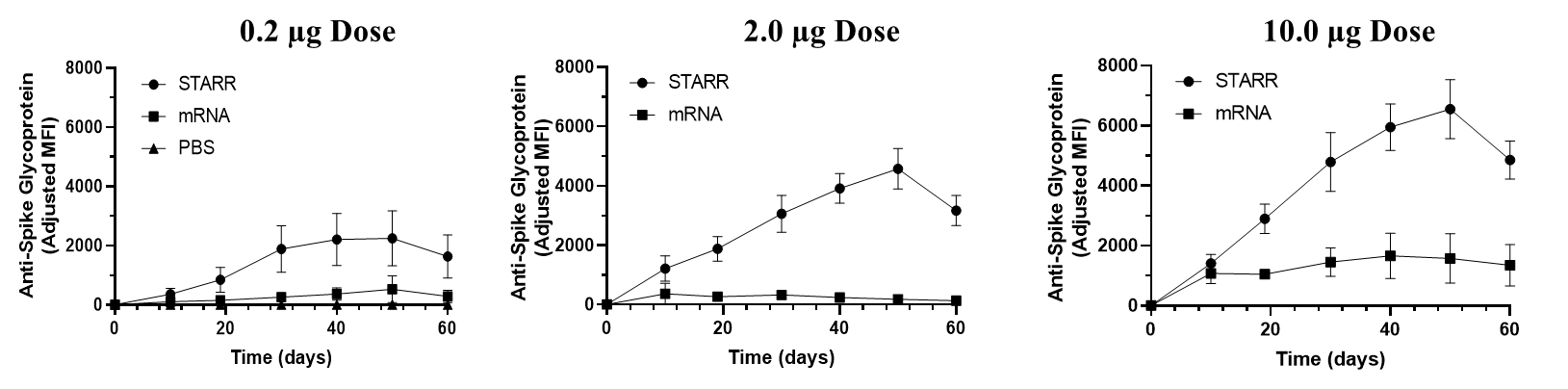
New preclinical data demonstrate that neutralising antibody levels in response to a single administration of LUNAR-COV19 (0.2, 2.0, 10.0 µg) continue to increase over 50 days. The increasing antibody levels are attributed to the self-replicating mRNA of LUNAR-COV19. (Image credit: Arcturus Therapeutics & Duke-NUS Medical School)
About Arcturus Therapeutics
Founded in 2013 and based in San Diego, California, Arcturus Therapeutics Holdings Inc. (Nasdaq: ARCT) is a clinical-stage mRNA medicines and vaccines company with enabling technologies: (i) LUNAR® lipid-mediated delivery, (ii) STARR™ mRNA Technology and (iii) mRNA drug substance along with drug product manufacturing expertise. Arcturus’ diverse pipeline of RNA therapeutic candidates includes programs to potentially treat Ornithine Transcarbamylase (OTC) Deficiency, Cystic Fibrosis, Glycogen Storage Disease Type 3, Hepatitis B, non-alcoholic steatohepatitis (NASH) and a self-replicating mRNA vaccine for SARS-CoV-2. Arcturus’ versatile RNA therapeutics platforms can be applied toward multiple types of nucleic acid medicines including messenger RNA, small interfering RNA, replicon RNA, antisense RNA, microRNA, DNA, and gene editing therapeutics. Arcturus’ technologies are covered by its extensive patent portfolio (192 patents and patent applications, issued in the U.S., Europe, Japan, China and other countries). Arcturus’ commitment to the development of novel RNA therapeutics has led to collaborations with Janssen Pharmaceuticals, Inc., part of the Janssen Pharmaceutical Companies of Johnson & Johnson, Ultragenyx Pharmaceutical, Inc., Takeda Pharmaceutical Company Limited, CureVac AG, Synthetic Genomics Inc., Duke-NUS, Catalent Inc., and the Cystic Fibrosis Foundation. For more information visit www.ArcturusRx.com
About Duke-NUS Medical School
Duke-NUS is Singapore’s flagship graduate entry medical school, established in 2005 with a strategic, government-led partnership between two world-class institutions: Duke University School of Medicine and the National University of Singapore (NUS). Through an innovative curriculum, students at Duke-NUS are nurtured to become multi-faceted ‘Clinicians Plus’ poised to steer the healthcare and biomedical ecosystem in Singapore and beyond. A leader in ground-breaking research and translational innovation, Duke-NUS has gained international renown through its five signature research programmes and nine centres. The enduring impact of its discoveries is amplified by its successful Academic Medicine partnership with Singapore Health Services (SingHealth), Singapore’s largest healthcare group. This strategic alliance has spawned 15 Academic Clinical Programmes, which harness multi-disciplinary research and education to transform medicine and improve lives.
For more information, please visit www.duke-nus.edu.sg
Forward Looking Statements
This press release contains forward-looking statements that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. Any statements, other than statements of historical fact included in this press release, including those regarding the Company’s expected performance, the Company’s development of any specific novel mRNA therapeutics, the Company’s efforts to develop a vaccine against COVID-19, and therapeutic potential thereof, based on the Company’s mRNA therapeutics, the forecasted safety, efficacy, characteristics or reliability of a vaccine against COVID-19, were one to be successfully developed based on the Company’s mRNA therapeutics, the dosing level and frequency of a vaccine against COVID-19 were one to be successfully developed based on the Company’s mRNA therapeutics and the impact of general business and economic conditions are forward-looking statements. Arcturus may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in any forward-looking statements such as the foregoing and you should not place undue reliance on such forward-looking statements. Such statements are based on management’s current expectations and involve risks and uncertainties, including those discussed under the heading "Risk Factors" in Arcturus’ Annual Report on Form 10-K for the fiscal year ended December 31, 2019, filed with the SEC on March 16, 2020 and in subsequent filings with, or submissions to, the SEC. No assurances can be given that any results reported in pre-clinical studies can be replicated in further studies or in human beings, or that a vaccine can or will ever be developed or approved using the Company’s technology. Except as otherwise required by law, Arcturus disclaims any intention or obligation to update or revise any forward-looking statements, which speak only as of the date they were made, whether as a result of new information, future events or circumstances or otherwise.
For media enquiries, please contact: