Back
Tuesday, 21 Apr, 2020
Duke-NUS scientists explore using ‘own’ immune cells to target infectious diseases including COVID-19
- Immunotherapy utilising own immune cells might also be useful in treating other difficult diseases, such as HIV, HBV, beside cancers
Singapore, 21 April 2020 – The engineering of specific virus-targeting receptors onto a patient’s own immune cells is now being explored by scientists from Duke-NUS Medical School (Duke-NUS), as a potential therapy for controlling infectious diseases, including the COVID-19-causing virus, SARS-CoV-2. This therapy that has revolutionised the treatment of patients with cancer has also been used in the treatment of other infectious diseases such as Hepatitis B virus (HBV), as discussed by the School’s researchers in a commentary published in the Journal of Experimental Medicine.
This therapy involves extracting immune cells, called T lymphocytes, from a patient’s blood stream and engineering one of two types of receptors onto them: chimeric antigen receptors (CAR) or T cell receptors (TCR). TCRs are naturally found on the surfaces of T lymphocytes while CARs are artificial T cell receptors that are generated in the laboratory. These receptors allow the engineered T lymphocytes to recognise cancerous or virus infected cells.
“This therapy is classically used in cancer treatment, where the lymphocytes of the patients are redirected to find and kill the cancer cells. However, its potential against infectious diseases and specific viruses has not been explored. We argue that some infections, such as HIV and HBV, can be a perfect target for this therapy, especially if lymphocytes are engineered using an approach that keeps them active for a limited amount of time to minimise potential side effects,” said Dr Anthony Tanoto Tan, Senior Research Fellow at the Duke-NUS’ Emerging Infectious Diseases (EID) programme and the lead author of this commentary.
This type of immunotherapy requires specialised personnel and equipment, and it needs to be administered indefinitely. This makes it cost-prohibitive for treating most types of viral infections. However, in the case of HBV infections, for example, current anti-viral treatments merely suppress viral replication and cure less than 5% of patients. Treating these patients with a combination of anti-virals and CAR/TCR T cells could be a viable option. The team’s approach using mRNA electroporation to engineer CAR/TCR T cells limits their functional activity to a short period of time, and hence provides enhanced safety features suited for its deployment in patients with chronic viral diseases.
“We demonstrated that T cells can be redirected to target the coronavirus responsible for SARS. Our team has now begun exploring the potential of CAR/TCR T cell immunotherapy for controlling the COVID-19-causing virus, SARS-CoV-2, and protecting patients from its symptomatic effects,” said Professor Antonio Bertoletti from the Duke-NUS’ EID programme, who is the senior author of this commentary.
“Infectious diseases remain a leading cause of morbidity and mortality worldwide, necessitating the development of novel and innovative therapeutics. Although immunotherapy is most commonly associated with the treatment of cancer or inflammatory diseases such as arthritis, this commentary accentuates the evolving role of this specialised treatment strategy for various infectious diseases,” said Professor Patrick Casey, Senior Vice Dean for Research at Duke-NUS.
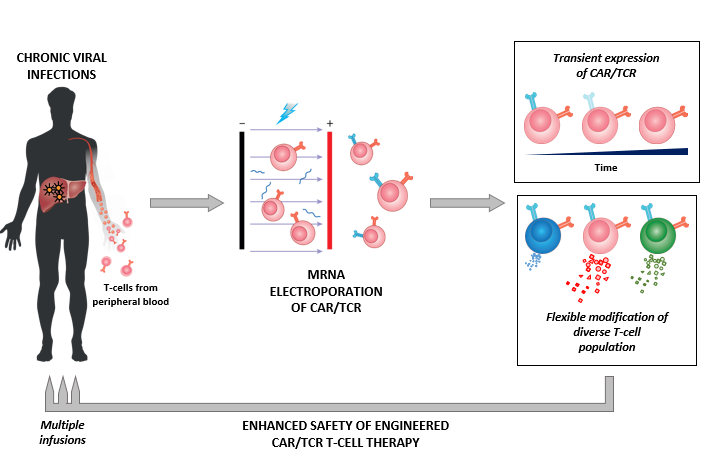
CAR/TCR T cells engineered through mRNA electroporation confers enhanced safety features suited for its deployment in patients with chronic viral diseases. (Image credit: Journal of Experimental Medicine)
Reference: Anthony Tanoto Tan and Antonio Bertoletti (2020). Challenges of CAR- and TCR-T cell–based therapy for chronic infections. Journal of Experimental Medicine.
Complete research paper available at this link: 10.1084/jem.20191663
For media enquiries, please contact Lekshmy Sreekumar, Duke-NUS Communications.